A Primer on mRNA COVID Vaccines
Answering your COVID-19 vaccine questions
A Primer on mRNA Covid-19 Vaccines

Thomas B. Casale, MD
FARE Medical Advisor
Food Allergy Research and Education (FARE)
Professor of Medicine and Pediatrics
Division of Allergy/Immunology
University of South Florida
Tampa, FL
What are the vaccines that will be approved first?
The vaccines produced by Pfizer, to be reviewed by the U.S. Food and Drug Administration (FDA) on December 10, and Moderna, to be reviewed by the FDA on December 17, will be the first two vaccines approved. Both should be approved this month, with distribution beginning within days of FDA approval, which should be noted is not a formal approval. The approval that will take place will be done through emergency use authorization which has a lower standard for formal approval.
How do these vaccines work?
These mRNA vaccines are a new type of vaccine platform designed to protect against COVID-19. Many of our current vaccines use an inactivated or weakened live virus or a purified protein from a virus that is injected into your body to induce an immune response against the virus. The two new vaccines use technology that is not new but has not been used before now to produce an antiviral vaccine approved for human use. mRNA vaccines use a type of genetic material, mRNA, to instruct our cells to make a whole viral protein or a piece of a protein that critical for the infectious nature of the virus. In the case of the SARS-CoV-2 virus that causes COVID-19, the mRNA vaccines contain the RNA instructions for making the spike protein, the little crown-shaped protein that gives coronavirus its name. Once the vaccine is injected into your arm, these RNA instructions cause your muscle cells to make pieces of spike protein that appear on the muscle cell surfaces. Your immune system recognizes that the spike proteins are foreign and mounts an immune response that includes making antibodies to target the foreign protein, just like the immune response to a natural coronavirus infection. After the spike protein piece is made, the muscle cell breaks down the mRNA instructions and gets rid of them within a few days. The mRNA is short-lived and does not change your own genetic code. After the second injection of the vaccine, your immune response is boosted, so that you can more efficiently and rapidly mount a vigorous immune response that protects you against the consequences of a natural infection. It is important to recognize that these mRNA vaccines cannot give you COVID-19. Below is an illustration of the vaccines’ mechanism of action, posted to NIH Director’s Blog on July 16 by the director of the National Institutes of Health, Dr. Francis Collins.
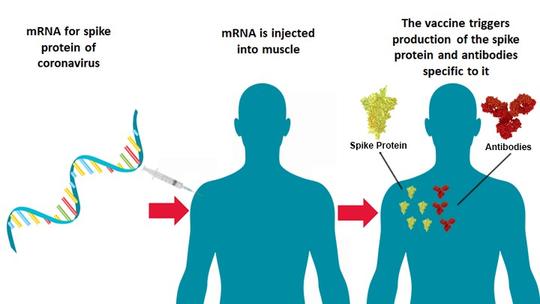
What is in the mRNA vaccines besides mRNA?
The main ingredients in mRNA vaccines are:
- An adjuvant, which stimulates the immune system to react more vigorously and increases the likelihood that the body will recognize the spike protein as “foreign,” is often contained in vaccines. Adjuvants are generally safe but designed to promote an inflammatory/immunologic response. The adjuvant is what causes most of the side effects that result from the vaccine including fever, headache, and pain and soreness in your muscles. So, these side effects are a sign that the vaccine is doing what it is supposed to. The Pfizer vaccine does not contain an adjuvant.
- Lipid (fat) and cholesterol nanoparticles (very small in size) that hold the mRNA and deliver it to muscle cells that use the mRNA to make the spike protein.
- Salts and sucrose (sugar).
The mRNA vaccines do not contain food proteins, including egg, and do not contain preservatives, including mercury.
How are the vaccines dosed?
The Pfizer COVID-19 Vaccine, BNT162b2 (30 μg), is administered intramuscularly (IM) as a series of two 30-μg doses (0.3 mL each) approximately 21 days apart. The Moderna vaccine will also require two doses administered IM approximately 28 days apart.
What are the typical side effects of the mRNA vaccines?
The most common adverse reactions reported for the Pfizer vaccine were injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%) and fever (14.2%). Severe adverse reactions occurred in 0.0% to 4.6% of participants, were more frequent after Dose 2 than after Dose 1 and were generally less frequent in participants older than 55 years of age (≤ 2.8%) as compared to younger participants (≤4.6%). Most adverse reactions tend to be mild to moderate in severity, last for 1 to 2 days, and improved with non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen (Tylenol). The frequency of serious adverse events was low (<0.5%) (https://www.fda.gov/media/144245/).
What about allergic reactions?
Pfizer’s most extensive safety trials showed allergies to the vaccine to be extremely rare (about 0.1%, or one per thousand, greater than the placebo: 0.63% vs. 0.51%). However, the clinical trials excluded individuals with a history of severe adverse reaction associated with a vaccine and/or severe allergic reaction (e.g., anaphylaxis) to any component of the study intervention(s). Out of thousands who received the vaccine on the United Kingdom’s first day of vaccinations, two people had allergic reactions from which they recovered. Both individuals had a history of severe allergic reactions in the past and carried epinephrine autoinjectors. At this time, it is not known whether these reactions can be attributed to the vaccine. It is important to remember that such reactions can happen with any vaccine or drug. Whether this will affect U.S. labeling and precautions for people with severe allergies will likely be determined during FDA deliberations. However, it is important to note that these vaccines do not contain egg, so there are no elevated specific risks for egg-allergic individuals.
How effective are the vaccines?
Both mRNA vaccines have an efficacy of close to 95%. The Pfizer data showed that 95% of individuals that received both doses of the vaccine were protected against natural coronavirus infection occurring at least 7 days after the second dose of vaccine. Subgroup analyses of the primary efficacy endpoint showed similar efficacy across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19 (https://www.fda.gov/media/144245/). A summary of the Pfizer data efficacy as listed by the FDA is:
- Reduction in the risk of confirmed COVID-19 occurring at least 7 days after Dose 2
- Reduction in the risk of confirmed COVID-19 after Dose 1 and before Dose 2
- Reduction in the risk of confirmed severe COVID-19 any time after Dose 1
Who will get the vaccine first?
The CDC has already recommended that the first group—designated as 1a—should include frontline health providers and support personnel. The 1a group likely should also include residents of nursing homes and other long-term care facilities.
How much will it cost to get the vaccine?
The vaccine will be free. However, some providers may still charge for administering the injection.
If someone already had COVID-19, should they still take the vaccine?
Scientists are saying that a vaccinated immunity should last longer than a naturally acquired immunity from an infection, suggesting the answer is yes.
Will the vaccine be available for young children?
It is likely that the first approvals for these vaccines will only be for individuals 18 years and above. Ongoing studies in the pediatric population will provide the necessary data for approval in younger individuals.
After I receive the vaccine, am I contagious?
No. Remember, these vaccines use only mRNA, not live viruses. You will not be contagious and will not have to quarantine.
Once I receive the vaccine, do I still need to wear a mask and practice social distancing?
Since the vaccine is not 100% effective and there are many unanswered questions remain (see below), it is likely that some recommendations for continued vigilance will be forthcoming from the Centers for Disease Control and Prevention (CDC).
What are some of the things we do not know about these two vaccines?
Since these vaccines are new there are many data gaps. Listed below are some of the key pieces of information that will likely be answered after more people are vaccinated and immunity is assessed over longer periods of time.
- Duration of protection: Currently, we do not have data on sustained efficacy over a period longer than a few months. It is likely that individuals will need booster immunizations, perhaps on an annual basis.
- Effectiveness in specific populations including:
- immunocompromised patients
- pediatric populations
- individuals previously infected with COVID-19
- Effectiveness against:
- asymptomatic infection
- transmission
- long-term effects of infection
- mortality
Are these the only two vaccines that will be available soon?
Scientists at AstraZeneca and the University of Oxford are first to publish their full data in a peer-reviewed scientific journal, The Lancet. Data confirm the vaccine is 70% effective and has a good safety profile. However, there are unanswered questions that could delay approval by the FDA until more studies are done.
Conclusion
I’m very optimistic about the effectiveness and relative lack of side effects of these new mRNA vaccines. When given the opportunity, I will be first in line to get vaccinated.


